Chapter: 20-24
wall mural: a large picture painted or affixed directly on a wall or ceiling.
coolant: a liquid or gas that is used to remove heat from something.
canister: a round or cylindrical container used for storing such things as food, chemicals, or rolls of film.
encounter: a meeting, especially one that happens by chance.
fishing tackle: fishing rods, lines, hooks, and bait.
tangle: twist together into a confused mass.
obsidian
pumice
A physical property is a characteristic that you can observe without changing or trying to change the composition of the substance. How something looks, smells, sounds, or tastes are all examples of physical properties.
Using Your Senses
To describe a sample of matter, you need to identify its state. Is the ride a solid, a liquid, or a gas? This property, known as the state of matter, is another physical property that you can observe.
If you multiply the width, height, and depth of the box together, you calculate the box’s volume. The volume of an object is the amount of space it occupies.
Weight is a measurement of force. Weight depends on the mass of the object and on gravity.
Density measures the amount of mass in a given volume. To calculate the density of an object, divide its mass by its volume. The density of water is the same in a glass as it is in a tub.
Solubility also does not depend on size. Solubility is the number of grams of one substance that will dissolve in 100 g of another substance at a given temperature.
Melting and boiling point also do not depend upon an object’s size. The temperature at which a solid changes into a liquid is called its melting point. The temperature at which a liquid changes into a gas is called its boiling point.
Some materials pull iron toward them. These materials are said to be magnetic. The lodestone is a rock that is naturally magnetic.
A chemical property is a characteristic that cannot be observed without altering the substance. Another way to define a chemical property, then, is the ability of a substance to undergo a change that alters its identity.
A physical change is one in which the form or appearance of matter changes, but not its composition.
Physical changes are occurring constantly. The sugar blending into the iced tea is an example of a physical change: dissolving.
Matter can change from any state to another. Freezing is the opposite of melting. During freezing, a liquid change into a solid. A liquid also can change into a gas. This process is known as vaporization. During the reverse process, called condensation, a gas changes into a liquid. In some cases, matter changes between the solid and gas states without ever becoming a liquid. The process in which a solid change directly into a gas is called sublimation. The opposite process, in which a gas changes into a solid, is called deposition.
During a chemical change, substances are changed into different substances. In other words, the composition of the substance changes.
Signs of Chemical Changes: color, energy (release or absorb), odor, gases or solids.
A solid that separates out of a solution during a chemical change is called a precipitate.
A meteoroid is a chunk of metal or stone in space. Every day, meteoroids enter Earth’s atmosphere. When this happens, the meteoroid burns as a result of friction with gases in the atmosphere. It is then referred to as a meteor or shooting star. The burning produces streaks of light. The burning is an example of a chemical change.
The substances produced during a chemical change cannot be changed back into the original substances by physical means.
In order to be recycled, wastes need to be physically—and sometimes chemically—changed.
During a chemical change, the form or the composition of the matter changes. The particles within the matter rearrange to form new substances, but they are not destroyed, and new particles are not created. The number and type of particles remains the same. As a result, the total mass of the matter is the same before and after a physical or chemical change. This is known as the law of conservation of mass.
hydrogen peroxide
ammonia
Sauerkraut is finely cut raw cabbage that has been fermented by various lactic acid bacteria.
Pellet fuels are biofuels made from compressed organic matter or biomass.
homogeneous mixtures
bouillon
A substance is matter that has the same fixed composition and properties. It can’t be broken down into simpler parts by ordinary physical processes, such as boiling, grinding, or filtering. Only a chemical process can change a substance into one or more new substances.
An element is an example of a pure substance; it cannot be broken down into simpler substances. Compounds also have fixed compositions. The ratio of the atoms in a compound is always the same.
Compounds also have fixed compositions. The ratio of the atoms in a compound is always the same.
Mixtures do not always contain the same proportions of the substances that they are composed of. Lemonade is a mixture that can be strong tasting or weak tasting, depending on the amounts of water and lemon juice that are added. It also can be sweet or sour, depending on how much sugar is added. But whether it is strong, weak, sweet, or sour, it is still lemonade.
A type of mixture where the substances are not mixed evenly is called a heterogeneous (he tuh ruh JEE nee us) mixture. The substances in a heterogeneous mixture are usually easy to tell apart, like the seeds from the fruit of a watermelon. Other examples of heterogeneous mixtures include a bowl of cold cereal with milk and the mixture of pens, pencils, and books in your backpack. A homogeneous (hoh muh JEE
nee us) mixture contains two or more substances that are evenly mixed on a molecular level but still are not bonded together. Another name for a homogeneous mixture is a solution.
The substance that dissolves—or seems to disappear—is called the solute. The substance that dissolves the solute is called the solvent.
Under certain conditions, a solute can come back out of its solution and form a solid. This process is called crystallization. Sometimes this occurs when the solution is cooled or when some of the solvent evaporates. Crystallization is the result of a physical change. When some solutions are mixed, a chemical reaction occurs, forming a solid. This solid is called a precipitate (prih SIH puh tayt). A precipitate is the result of a chemical change.
Stalactites (hanging) and stalagmites (piles on the bottom) form as more rock accumulates.
First, minerals dissolve in water as it flows through rocks at the top of the cave. This solution of water and dissolved minerals drips from the ceiling of the cave. When drops of the solution evaporate from the roof of the cave, the minerals are left behind. They create the hanging rock formations called stalactites. When drops of the solution fall onto the floor of the cave and evaporate, they form stalagmites. Very often, a stalactite develops downward while a stalagmite develops upward until the two meet. One continuous column of minerals is formed.
Carbonated beverages are liquid-gas solutions—carbon dioxide is the gaseous solute, and water is the liquid solvent. The carbon dioxide gas gives the beverage its fizz and some of its tartness.
In a liquid-liquid solution, both the solvent and the solute are liquids. Vinegar, which you might use to make salad dressing, is a liquid-liquid solution made of 95 percent water (the solvent) and 5 percent acetic acid (the solute).
In gaseous solutions, a smaller amount of one gas is dissolved in a larger amount of another gas. This is called a gas-gas solution because both the solvent and solute are gases. The air you breathe is a gaseous solution.
In solid solutions, the solvent is a solid. The solute can be a solid, liquid, or gas. The most common solid solutions are solid-solid solutions—ones in which the solvent and the solute are solids. A solid-solid solution made from two or more metals is called an alloy. It’s also possible to include elements that are not metals in alloys. For example, steel is an alloy that has carbon dissolved in iron. The carbon makes steel much stronger and yet more flexible than iron.
A solution in which water is the solvent is called an aqueous (A kwee us) solution. Because water can dissolve so many different solutes, chemists often call it the universal solvent.
When certain atoms form compounds, they share electrons. Sharing electrons is called covalent
bonding. Compounds that contain covalent bonds are called molecular compounds, or molecules.
Some atoms do not share electrons when they join with other atoms to form compounds. Instead, these atoms lose or gain electrons. When they do, the number of protons and electrons within an atom are no longer equal, and the atom becomes positively or negatively charged. Atoms with a charge are called ions. Bonds between ions that are formed by the transfer of electrons are called ionic bonds, and the compound that is formed is called an ionic compound.
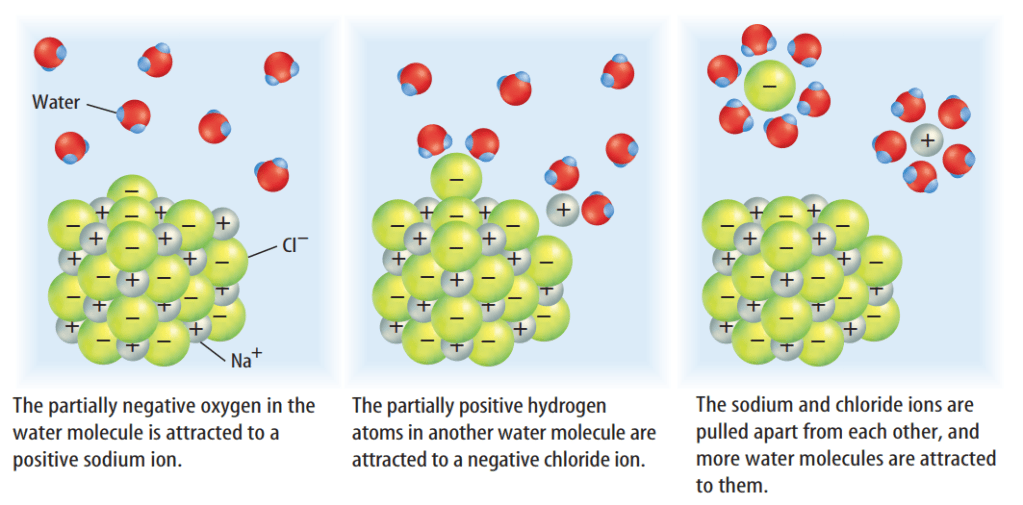
Water simply moves between different molecules of sugar, separating them. Like water, a sugar molecule is polar. Polar water molecules are attracted to the positive and negative portions of the polar sugar molecules. When the sugar molecules are separated by the water and spread throughout it, they have dissolved.
soluble
insoluble
When trying to predict which solvents can dissolve which solutes, chemists use the rule of “like dissolves like.” This means that polar solvents dissolve polar solutes and nonpolar solvents dissolve nonpolar solutes. In the case of sugar and water, both are made up of polar molecules, so sugar is soluble in water. In the case of salt and water, the sodium and chloride ion pair is like the water molecule because it has a positive charge at one end and a negative charge at the other end. On the other hand, if a solvent and a solute are not similar, the solute won’t dissolve. For example, oil and water do not mix. Oil molecules are nonpolar, so polar water molecules are not attracted to them.
Solubility (sahl yuh BIH luh tee) is a measurement that describes how much solute dissolves in a given amount of solvent. The solubility of a material has been described as the amount of the material that can dissolve in 100 g of solvent at a given temperature.
In liquid solutions, The solubility of many solutes changes if you change the temperature of the solvent. For example, if you heat water, not only does the sugar dissolve at a faster rate, but more sugar can dissolve in it. Unlike liquidsolid solutions, an increase in temperature decreases the solubility of a gas in a liquid-gas solution.
If you add calcium carbonate to 100 g of water at 25°C, only 0.0014 g of it will dissolve. Additional calcium carbonate will not dissolve. Such a solution—one that contains all of the solute that it can hold under the given conditions—is called a saturated solution. A hot solvent usually can hold more solute than a cool solvent can. When a saturated solution cools, some of the solute usually falls out of the solution. But if a saturated solution is cooled slowly, sometimes the excess solute remains dissolved for a period of time. Such a solution is said to be supersaturated, because it contains more than the normal amount of solute. Solubility does not tell you how fast a solute will dissolve—it tells you only how much of a solute will dissolve at a given temperature.
A solute dissolve faster when the solution is stirred or shaken or when the temperature of the solution is increased. Molecules are always moving and colliding. The collisions must take place for chemical processes to occur. The chemical processes take place at a given rate of reaction. Temperature has a large effect on that rate. The higher the temperature, the more collisions occur and the higher the rate of reaction. The opposite is also true. The lower the temperature, the less collisions occur and the lower the rate of reaction.
The concentration of a solution tells you how much solute is present compared to the amount of solvent. You can give a simple description of a solution’s concentration by calling it either concentrated or dilute.
One way of giving the exact concentration is to state the percentage of the volume of the solution that is made up of solute.
All solute particles affect the physical properties of the solvent, such as its boiling point and freezing point. The effect that a solute has on the freezing or boiling point of a solvent depends on the number of solute particles.
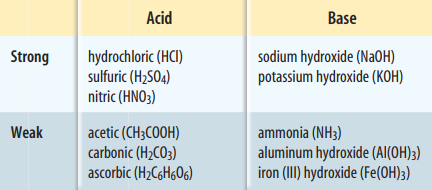
Acids are substances that release positively charged hydrogen ions, H+, in water. When an acid mixes with water, the acid dissolves, releasing a hydrogen ion. The hydrogen ion then combines with a water molecule to form a hydronium ion. Hydronium ions are positively charged and have the formula H3O+.
Many acids can cause serious burns to body tissues. Acidic solutions also are corrosive, which means
they can break down certain substances. Many acids can corrode fabric, skin, and paper. The solutions of some acids also react strongly with certain metals. The acid-metal reaction forms metallic compounds and hydrogen gas, leaving holes in the metal in the process.
Acidic solutions can conduct electricity.
Citric acid is an organic compound with the chemical formula HOC(CH₂CO₂H)₂. It is a colorless weak organic acid. It occurs naturally in citrus fruits.
Vitamin C, also known as ascorbic acid, has several important functions. These include: helping to protect cells and keeping them healthy. maintaining healthy skin, blood vessels, bones and cartilage.
Formic acid (from Latin formica ‘ant’), systematically named methanoic acid, is in use as a preservative and antibacterial agent, it is also useful in the manufacturing of leather and rubber. It is useful as a miticide by beekeepers.
Sulfuric acid is used in the production of fertilizers, steel, paints, and plastics.
Hydrochloric acid, which is known commercially as muriatic acid, is used in a process called pickling. Pickling is a process that removes impurities from the surfaces of metals. Hydrochloric acid also can be used to clean mortar from brick walls.
Nitric acid is used in the production of fertilizers, dyes, and plastics.
Acids often are used in batteries because their solutions conduct electricity. For this reason, it sometimes is referred to as battery acid.
Carbonic acid plays a key role in the formation of caves and of stalactites and stalagmites. Carbonic acid is formed when carbon dioxide in soil is dissolved in water. When this acidic solution comes in contact with calcium carbonate—or limestone rock—it can dissolve it, eventually carving out a cave in the rock.
Bases are substances that can accept hydrogen ions. When bases dissolve in water, some hydrogen atoms from the water molecules are attracted to the base. A hydrogen atom in the water molecule leaves behind the other hydrogen atom and oxygen atom. This pair of atoms is a negatively charged ion called a hydroxide ion. A hydroxide ion has the formula OH. Most bases contain a hydroxide ion, which is released when the base dissolves in water.
For example, sodium hydroxide is a base with the formula NaOH. When NaOH dissolves in water, a sodium ion and the hydroxide ion separate.
Acids in water solution taste sour but bases taste bitter—as you know if you have ever accidentally gotten soap in your mouth. Like acids, bases are corrosive. Bases can cause burns and damage tissue. Basic solutions are not as reactive with metals as acidic solutions are.
Lye
lime
pH is a measure of how acidic or basic a solution is. The pH scale ranges from 0 to 14. Acidic solutions have pH values below 7. A solution with a pH of 0 is very acidic. Hydrochloric acid can have a pH of 0. A solution with a pH of 7 is neutral, meaning it is neither acidic nor basic. Pure water is neutral. Basic solutions have pH values above 7. A solution with a pH of 14 is very basic. Sodium hydroxide can have a pH of 14.
The pH of a solution is related directly to its concentrations of hydronium ions (H3O) and hydroxide ions (OH). Acidic solutions have more hydronium ions than hydroxide ions. Neutral solutions have equal numbers of the two ions. Basic solutions have more hydroxide ions than hydronium ions.
To determine the difference in pH strength, use the following calculation: 10n, where n the difference between pHs. For example: pH3-pH1=2, 10^2=100 times more acidic.
The strength of an acid is related to how easily the acid separates into ions, or how easily a hydrogen ion is released, when the acid dissolves in water. Similarly, the strength of a base is related to how easily the base separates into ions, or how easily a hydroxide ion is released, when the base dissolves in water.
Indicators are compounds that react with acidic and basic solutions and produce certain colors, depending on the solution’s pH. Some indicators, such as litmus, are soaked into paper strips. When litmus paper is placed in an acidic solution, it turns red. When placed in a basic solution, litmus paper turns blue.
Neutralization (new truh luh ZAY shun) is the reaction of an acid with a base. It is called this because the properties of both the acid and base are diminished, or neutralized. In most cases, the reaction produces a water and a salt.
When one hydronium ion reacts with one hydroxide ion, the product is two water molecules. This reaction occurs during acid-base neutralization. Equal numbers of hydronium ions from the acidic solution and hydroxide ions from the basic solution react to produce water. Pure water has a pH of 7, which means that it’s neutral.
agitate or wiggle: shake or stir
Gargled salt water is a disinfectant; it fights the bacteria that cause some sore throats.
Matter is anything that takes up space and has mass. Matter doesn’t have to be visible—even air is matter.
All matter is made up of tiny particles, such as atoms, molecules, or ions. Each particle attracts other particles. In other words, each particle pulls other particles toward itself. These particles also are constantly moving. The motion of the particles and the strength of attraction between the particles determine a material’s state of matter.
There are three familiar states of matter—solid, liquid, and gas. A fourth state of matter known as plasma occurs at extremely high temperatures.
A solid is matter with a definite shape and volume. A solid’s particles are vibrating in place.
In some solids, the particles are arranged in a repeating, three-dimensional pattern called a crystal. These solids are called crystalline solids. However, Some solids come together without forming crystal structures. The particles are found in a random arrangement. These solids are called amorphous (uh MOR fuhs, from Latin a- ‘without’ + morphē ‘form’) solids. Rubber, plastic, and glass are examples of amorphous solids.
A liquid is matter that has a definite volume but no definite shape. The particles in a liquid have enough energy to move out of their fixed positions but not enough energy to move far apart.
molasses: thick, dark brown syrup obtained from raw sugar during the refining process, a version of which is used in baking.
Some liquids flow more easily than others. A liquid’s resistance to flow is known as the liquid’s viscosity (means sticky). Honey has a high viscosity. Water has a lower viscosity. The viscosity results from the strength of the attraction between the particles of the liquid. For many liquids, viscosity increases as the liquid becomes colder.
The uneven forces acting on the particles on the surface of a liquid are called surface tension. Surface tension causes the liquid to act as if a thin film were stretched across its surface.
Gas is matter that does not have a definite shape or volume. Water vapor is the term for the gas state of water.
The energy of motion is called kinetic energy.
The energy of motion is called kinetic energy. Particles within matter are in constant motion. The amount of motion of these particles depends on the kinetic energy they possess. Particles with more kinetic energy move faster and farther apart. Particles with less energy move more slowly and stay closer together. Potential energy is the energy that is stored in an object due to its position relative to some zero position.
The total kinetic and potential energy of all the particles in a sample of matter is called thermal energy. Thermal energy, an extensive property, depends on the number of particles in a substance as well as the amount of energy each particle has.
The average kinetic energy of the individual particles is the temperature, an intensive property, of the substance.
The movement of thermal energy from a substance at a higher temperature to one at a lower temperature is called heat.
The specific heat of a substance is the amount of heat required to raise the temperature of 1 g of a substance 1°C.
mushy: soft and pulpy.
The change from the liquid state to the solid state is called freezing. The change from the
solid state to the liquid state is called melting.
The temperature at which a substance changes from the liquid state to the solid state is called the freezing point. The freezing point of the liquid state of a substance is the same temperature as the melting point of the solid state.
The change from a liquid to a gas is known as vaporization(vay puh ruh ZAY shun).
Vaporization that takes place at the surface of a liquid is called evaporation. Vaporization that takes place below the surface of a liquid is called boiling.
It takes more than speed for water molecules to escape the liquid state. During evaporation, these faster molecules also must be near the surface, heading in the right direction, and they must avoid hitting other water molecules as they leave. With the faster particles evaporating from the surface of a liquid, the particles that remain are the slower, cooler ones. Evaporation cools the liquid and anything near the liquid. You experience this cooling effect when perspiration evaporates from your skin.
As a gas cools, its particles slow down. When particles move slowly enough for their attractions to bring them together, droplets of liquid form. This process, which is the opposite of vaporization, is called condensation. As a gas condenses to a liquid, it releases the thermal energy it absorbed to become a gas.
Some substances can change from the solid state to the gas state without ever becoming a liquid. During this process, known as sublimation, the surface particles of the solid gain enough energy to become a gas.
Pressure is equal to the force exerted on a surface divided by the total area over which the force is exerted. A more useful unit when discussing atmospheric pressure is the kilopascal (kPa), which is 1,000 pascals. Atmospheric pressure is 101.3 kPa at sea level.

Altitude is the height above sea level. As altitude increases atmospheric pressure decreases. This is because fewer air particles are found in a given volume. Fewer particles have fewer collisions, and therefore exert less pressure.
If the temperature of a confined gas increases, the pressure of the gas will increase. As volume
decreases, pressure increases.
The difference in pressure results in an upward force on an object immersed in a fluid. This force is known as the buoyant force. If the buoyant force is equal to the weight of an object, the object will float. If the buoyant force is less than the weight of an object, the object will sink. Weight is a force in the downward direction. The buoyant force is in the upward direction. An object will float if the upward force is equal to the downward force.
According to Archimedes’ (ar kuh MEE deez) principle, the buoyant force on an object is equal to the weight of the fluid displaced by the object.
Density is mass divided by volume.
When a force is applied to a confined fluid, an increase in pressure is transmitted equally to all parts of the fluid. This relationship is known as Pascal’s principle.
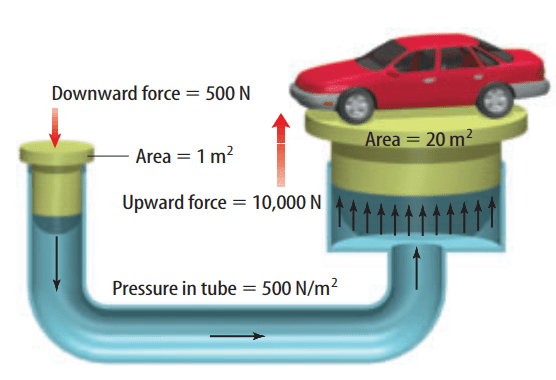
If an otherwise closed container has a hole in it, any fluid in the container will be pushed out the opening when you squeeze it. This arrangement, known as a force pump, makes it possible for you to squeeze toothpaste out of a tube or mustard from a plastic container.
ramp: slope

Leave a comment